Aptima® Sexual and Vaginal Health Solutions Test
Rely on excellent sensitivity and specificity for your sexual and vaginal health testing needs.

Overview
Documents
Training
The most comprehensive sexual and vaginal health molecular menu.
Hologic offers the most comprehensive sexual and vaginal health portfolio that allows you to automate molecular testing workflows while instilling confidence in your clinical results.
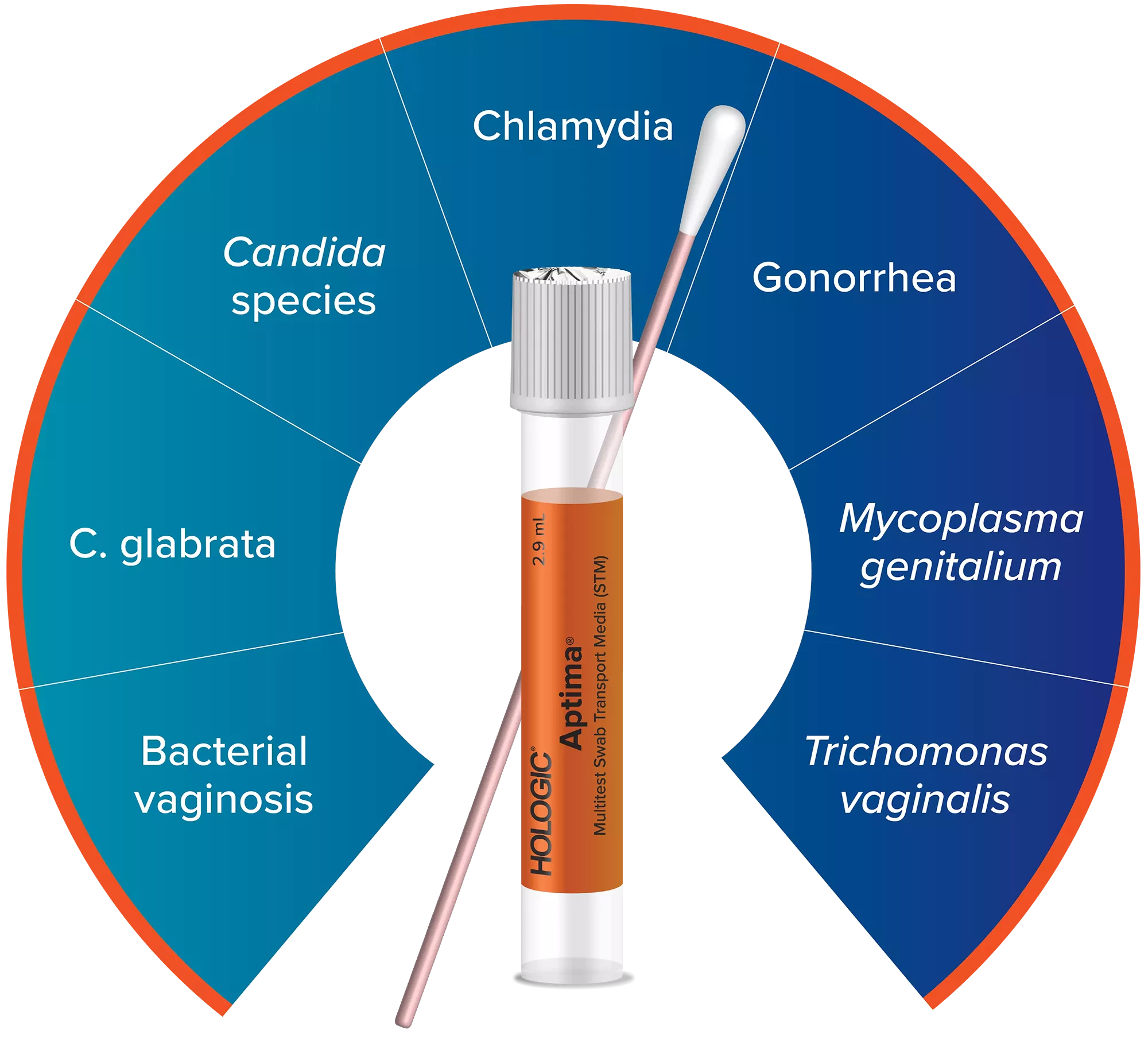
One Sample, Multiple Results
With innovative nucleic acid amplification test (NAAT) and a single collection device, the Aptima® Multitest Swab simplifies testing by helping to detect up to 7 infections and disease states from just 1 vaginal swab.1-5
Expert Partner
Through continuous clinical research and trusted testing solutions, Hologic supports our partners in the fight against sexually transmitted infections (STIs) and accurate detection of vaginitis.
Proven Performance
Our sexual and vaginal health portfolio leverages best-in-class technology to provide timely and reliable results to patients.
Streamlined Workflow
The Aptima sexual and vaginal health portfolio can run alongside multiple disease areas on our fully automated Panther® Scalable Solutions.
Evolve Your Offerings with Aptima Assays
7 infections and disease states
can be detected with the Aptima sexual and vaginal health portfolio to support your testing needs.
35 years of experience
in sexual and vaginal health molecular testing and helping stop the spread of disease.
A Comprehensive, Flexible Menu
By expanding access to timely and accurate results, Hologic empowers patients to protect their sexual and reproductive health.
Aptima Combo 2® assay (for chlamydia / gonorrhea)
Detects chlamydia and gonorrhea from a single sample. By using this highly specific and sensitive test, laboratories can improve workflow while allowing healthcare professionals to identify and treat patients with these often-asymptomatic infections to prevent long-term health consequences.1
Aptima® Mycoplasma genitalium assay
When identifying Mycoplasma genitalium, the test you choose matters. A highly sensitive rRNA test is needed for an accurate diagnosis, and NAAT is the most effective way to identify this STI. Hologic developed the first FDA-cleared sample-to-result test to detect this highly prevalent STI.2

The Significance of Diagnostic Testing in Women’s Health: Mycoplasma genitalium
Dr. Kyle Bukowski*, Chief Medical Officer for Planned Parenthood of Maryland, discusses real-world data reflective of current practices in the U.S. for Mycoplasma genitalium.
The Latest News on Aptima Sexual and Vaginal Health
Increasing Certainty in Sexual & Vaginal Health
The Aptima sexual and vaginal health portfolio represents a comprehensive menu to champion women’s health worldwide. By supporting sexual health testing with innovative technology and a fully automated, sample-to-result platform, we are helping advance early detection and treatment.
Hologic is committed to protecting sexual and reproductive health by preventing the spread of STIs and vaginitis. We are continuously investing in R&D efforts designed to deliver new products to help healthcare providers and laboratories provide better patient care, now and in the future.